Pre-empting the Lord of the Lungs
- The Mischievous Stethoscope
- Dec 7, 2020
- 12 min read
Lung cancer is deemed to be a disease of high mortality and morbidity, where the standardised incidence and mortality rates are respectively at 23.1 and 19.7 in 100,000 people. It is, globally, the greatest contributor of new cancer diagnoses, accounting for 12.4 per cent. Survival outcomes remain poor due to the fact that most patients present with advanced stage of disease, where palliative therapy is usually the only option. [1-2] Smoking is a major risk factor of this debilitating disease. Breaking it down to histological subtype, male smokers who have a daily dose of > 30 cigarettes, report whopping odds ratios of 103.5 for squamous cell carcinomas and 111.3 for small cell lung carcinomas (which is the subtype of the lowest survival rate and worst prognostic outcomes). Female smokers report odds ratios of 62.7 and 108.6 respectively. [3]
Regarding the rise of screening in different types of cancer, not less promoted by cervical cancer screening campaigns (it doesn't stop here, we've got cervical smear tests and HPV vaccination for both men and women...it's a revolution), different research groups around the world such as the National Lung Screening Trial initiated by the National Cancer Institute in the US [4], and the NELSON trial in Belgium and the Netherlands, advocate early screening protocols amongst heavy smokers for lung cancer. This is expected to pick up early signs and manifestations of the disease, therefore nipping the bud before it has the chance to grow. In other words, adopting the tone of adult fantasies, we are effectively 'pre-empting the Lord of the Lungs'. Lord of the Lungs, because lung cancer, once taken form and root, calls the shots and rules its empire with verve and vigour. If we can't control the primary lesion in the lung, there is an even smaller chance for us to check its spread across the thorax and the rest of the body.
Although it seems very rational to propose a screening programme of this sort, considering the heavy burden lung cancer poses to healthcare systems worldwide, we must also be very cautious. Just as we've explored in prostate cancer, over-diagnosis of clinically insignificant lesions (in the case of lung cancer, there are rarely clinically insignificant lesions; however, there can be higher rate of detection of benign lung lesions which can be mistaken as cancer, such as harmatomas and pulmonary arteriovenous malformations) can lead to over-treatment. The patient having received over-treatment is more likely to die of the treatment rather than the lesion itself. Balancing exercises have to be executed before proceeding. Logical deduction shall not prevail over meticulous contemplation and planning. Also, what should we use to screen for lung cancer: the standard Chest X-Ray which is more clinically available and carries less radiation, or the more accurate CT?
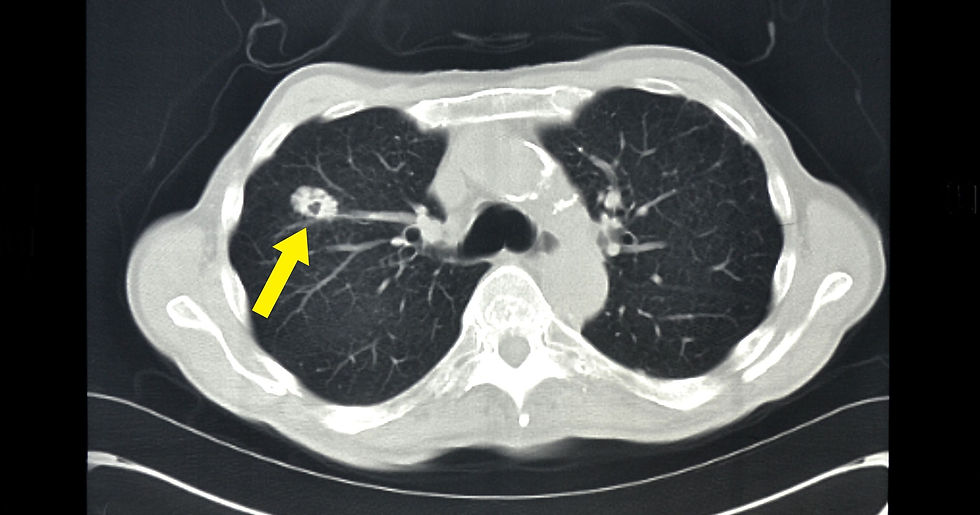
Axial CT plus contrast showing lung cancer on the right lung (extracted from: https://www.gannett-cdn.com/-mm-/ed76d4b30ab54f46bba2dda3982cd66de319ac30/c=0-186-3665-2257/local/-/media/2015/01/23/LAGroup/LafayetteLA/635576255612195406-CT-slice-LungCancer.jpg?width=3200&height=1680&fit=crop); there is also a tiny speck in the middle (hypoattenuation as compared to the surrounding tissue) indicative of cavitation with thick walls surrounding it - likely to be squamous cell carcinoma of the lung.

Coronal CT plus Contrast showing lung cancer in the left upper lobe, obstructing the left main bronchus, leading to reduced left lung volume (compare this with the right lung) (Courtesy of Radiopaedia)

Chest X-Ray (Posterior-Anterior or PA) showing a lung cancer on the left lung between ribs four to seven, which is heterogenous in density and has a jagged contour (courtesy of Radiopaedia). The lateral film needs to be consulted to see if the lesion is tethered to the pericardium (the outer covering of the heart) and/or the descending aorta.
Let's dive in and explore the results of the NELSON trial, published recently on New England Journal of Medicine*. [5]
The Paper
Recruitment of Participants: It has to be stressed that this study has initially predominantly recruited male participants, due to the higher prevalence of heavy smoking in the male population. However, later on, female participants were also recruited, albeit still at a substantially lower proportion than males. Moreover, this study focuses on patients who smoke heavily - defined as > 15 cigarettes daily for 25 years, or > 10 cigarettes daily for 30 years. Both current and former smokers (those who have quitted >=10 years ago) are included. Heavy smoking, as stated above, is a major risk factor. However, it is not the only risk factor. Family history and presence of other lung diseases (such as bronchiectasis and cystic fibrosis) are also possible risk factors of lung cancer. Pulmonary fibrosis, for instance, operates through the chronic inflammatory route. Further studies have to be executed to see if routine, annual screening should be extended to these patient groups.
Two Patient Groups: Those receiving CT Screening and those without (control group);
Exclusion Criteria:
Patients reporting moderate or severe health problems, and an inability to climb two flights of stairs (general health status assessment - this study focuses on screening; if the patient is receiving treatment for other diseases, it means two things - a. the medication might confound the risk of lung cancer and its detection; b. the underlying condition might affect the risk of lung cancer and its detection);
Body Weight > 140 kg (this can be due to concerns of reduced accuracy in detection since body fat and its distribution, if too centrally-located and in excess, may interfere with the radiation);
Current/Past melanoma, renal cancer or breast cancer (confounding- metastases and recurrent cancer);
Diagnosis of Lung Cancer or treatment related to lung cancer for the past 5 years (I suppose this time limit is rather arbitrary but I do appreciate the fact that the longer the relapse-free survival period, the lower the probability that the newly-diagnosed cancer event is related to the former one);
Patients who have received a chest CT Scan within the past year (some lesions may already be detected and treated by then- this affects the results of the trial).
CT Technique: Unlike the National Lung Screening Trial in the US, this trial uses a volume-based technique where the nodule detected has its volume, rather than diameter measured and graded. This is believed to be more accurate since it gives a more holistic view of the lesion. Moreover, it reduces the chance of over-diagnosis and over-treatment. The decision to NOT administer contrast in this trial may also be related to the inherent risks of contrast use (iodine-based contrast medium for CT can lead to renal failure, hepatotoxicity and lung fibrosis) and the quest for higher clinical efficiency.
Logistics for Screening: 4 Times: Baseline (Start of Trial), Year 1, Year 2 and Year 2.5; Follow-up: Year 5, Year 7 and Years 10-11 (the final one is carried out between Years 10 and 11);
Primary Outcome: Lung Cancer-specific Mortality, where this is determined by an expert committee, with the international mortality advisory committee having decided that the biases are relatively small.

[5]
From the above table, we can see that there are no significant differences between the two groups in terms of age and smoking habits, duration and quantity (the number of cigarettes smoked per day). This is very encouraging since it means we don't have to worry too much about extrinsic factors confounding the results of the trial.
Results: We focus on the grading of the cancer received upon diagnosis, lung cancer-related incidence and lung cancer-specific mortality amongst the two groups. In terms of histological subtype, there isn't much discussion in the paper but I postulate that some histological subtypes are easier to be detected. This correlates with the molecular and phenotypic features of different cancers. For example, squamous cell lung carcinomas tend to be more centrally located and feature cavitation, whereas lung adenocarcinomas, peripheral and solid. [6]

[5]
As we can see from Figure 1, there is a sharp increase in lung cancer incidence in the Screening Group in the first two years, where the trend then follows a downward trajectory (albeit slight). In general, the incidence of the Control Group increases throughout the monitoring period. The lung cancer incidence of the Screening Group is consistently higher than that of the Control Group. However, this is to be appreciated as a natural consequence of screening since it picks up more cases of malignant transformation - this allows us to introduce intervention and curative treatment at an earlier stage.
As for lung cancer-specific mortality, both groups show upward trajectories, with the Control Group's mortality statistic overtaking that of the Screening Group in between the first and second years after randomisation. The fact that the Screening Group bears higher mortality at first (from point of randomisation to the point between first and second years) may be attributed to the increased detection of life-threatening lesions in those patients. Up to the tenth year after randomisation, for the Screening Group, there are 2.50 deaths per 1000 person-years. For the Control Group, there are 3.30 deaths per 1000 person-years.
It has to be stated, however, that the all-cause mortality rates for both groups are similar - 13.93 for the Screening Group and 13.76 for the Control Group. The all-cause mortality rates refer to general death rates, regardless of lung cancer. I think their inclusion is important here to see if CT screening itself promotes more harm than good.

[5]
Screening Group: Most lung cancers detected by screening are of stages IA and IB (accounting for a total of 58.6 per cent), while non-screening-detected lung cancers are mostly in stage IV (accounting for 51.8 per cent).
Control Group: Almost half of lung cancers detected in this case are at stage IV (45.7 per cent).
This confirms the long-held belief that screening helps us pick up early-stage diseases so that we can initiate treatment, mostly curative surgery, as soon as possible, improving patient outcomes and prognosis. Lung cancer, if not screened routinely, is only picked up at the advanced stage where the only palatable option is palliation.
In terms of the histological subtype, while it can be due to the presence of distinctive radiological features in some that reflect their higher prevalence in this cohort (as mentioned, for instance, adenocarcinomas are more solid and peripheral), I think it boils down to the general prevalence of the subtype in the community.** In a Spanish study, 85.8 per cent of patients were diagnosed of non-small cell lung carcinoma and the most common reported subtype was the adenocarcinoma, at 71.4 per cent. [7] In an Indian study, the prevalence rates of adenocarcinomas and squamous cell carcinomas are roughly the same, at 36.4 per cent. [8] In Brazil, a similar phenomenon can be observed. Adenocarcinomas and squamous cell carcinomas are the most prevalent histological subtypes in the cohort, at respectively 43.3 per cent and 36.5 per cent. [9] Across all groups shown in Table 3, adenocarcinomas and squamous cell carcinomas are the most prevalent. The role of CT screening is thus conceived to have a minimal effect here.
Four Extra Points:
It is encouraging to see people being so health-conscious although they still smoke (well, to be fair, current non-smokers are also invited to the trial) - at least 87.6 per cent of participants underwent three screenings. It indicates that apart from screening, we can do more in promoting smoking cessation, such as promulgating the components of the WHO policy package MPOWER***. [10]
A significant benefit is shown in the analysis of the small subgroup of female subjects with respect to lung cancer-specific mortality. This concurs with the findings of the German Lung Cancer Screening Intervention Trial. It is unlikely that this difference is associated with the female sex hormone oestrogen, since it is found to be promoting the growth, development and spread of lung cancer. Female individuals (post-menopausal) taking hormonal replacement therapy also report higher incidence rates of lung cancer (relative risk: 1.26). [11-12]
In terms of overdiagnosis, the trial reports 19.7 per cent. However, extending the follow-up period to 11 years after randomisation, the rate drops to 8.9 per cent. This means that CT screening can have a lead time as long as 9 to 12 years for some cancers, effectively detecting them while they're still at their buds. Such cancers are expected to take more time to grow;
Although not explicitly discussed in the trial, I think this is worth mentioning - cynics might say that CT Screening leads to an increase in healthcare expenditure and undermines the cost-effectiveness of national healthcare providers such as the NHS in the UK. However, we need to bear in mind that screening is primarily targeted at those with history of heavy smoking. Although CT might sound a bit dramatic, it isn't in the long-term. Treatment, monitoring and palliation all come with hefty bills. This especially rings true for lung cancer targeted therapies. Early detection and treatment improve outcomes for patients and reduce the burden of the disease on the national healthcare system effectively.


Both Scans taken from the same patient (Courtesy of Radiopaedia)- Coronal CT Scans of the same stack showing a right upper lobe lesion that invades both the mediastinum (central portion of the scan) and the hilum (below). The black conduits in the second scan indicate the trachea and main bronchi at the carina (T4). The superimposed opacity is the upper lobe lesion in question. Upon histological examination, the diagnosis of small Cell Lung Carcinoma is made. Since there are no signs the other hemithorax is affected, this would be limited stage disease.
Brief Note on the National Lung Screening Trial (NLST) [13]:
The key difference, apart from the quantification of the lesion detected on CT, between the two trials is that for the NLST, it compares those who are screened by Chest X-Ray and CT respectively, thereby comparing the two modalities in terms of efficacy in lesion detection.
Adherence Rate: >90 percent (higher than the NELSON Trial);
Incidence of Lung Cancer: 645 cases per 100,000 people (CT Group), 572 cases per 100,000 people (Chest X-Ray Group);
Death Rates (lung cancer-specific): 247 cases per 100,000 people (CT Group), 309 cases per 100,000 people (Chest X-Ray Group), where the relative reduction in mortality (lung cancer-specific) with CT Screening is 20.0 per cent;
The cohort is much greater in the NLST than the NELSON: 53,454 in the former and 15,789 in the latter.
The study concludes that CT is the modality of choice in screening. However, do bear in mind that there are methodological differences between NLST and NELSON, even though they both advocate CT Screening in heavy smokers.

[13] I've highlighted some key statistics regarding the detection of lung cancer lesions by CT and X-Ray in the NLST. As we can see, those who present with positive screening tests in the CT Group mostly have Stage IA lesions (over half). This figure drops to 32.7 per cent in the Chest X-Ray Group. A significant percentage in the Chest X-Ray Group presenting with positive screening tests, also have Stage IV (Advanced) cancer. Comparing those reporting positive screening tests with those reporting negative ones across both CT and Chest X-Ray Groups, greater proportions of patients presenting with positive screening tests have low-grade disease. This tells us that screening, regardless of imaging modality, does work. Across both groups, the combined prevalence of adenocarcinomas and squamous cell carcinomas is greater than that of any other histological subtype. This concords with the results shown in the NELSON trial. However, for those in the CT Group reporting negative screening tests, small cell lung carcinoma accounts for a whopping 34.1 per cent and is the single most prevalent histological subtype in that subgroup. This might stoke fears of CT not being able to detect small cell lung carcinomas effectively, in contrast to other histological subtypes.
It is time for us to be more alert to lung cancer. Any attempt to pre-empt the Lord of the Lungs is naturally welcome. Recently published results from the NELSON Trial, as well as past trials such as the NLST, offer us new opportunities that can no longer be ignored.
*You might be wondering why I'm always discussing papers published by the NEJM. The truth is, I've changed my subscription from The Lancet to NEJM. Naturally, to get the most out of my subscription, I lean towards reading the latter although, to be frank, I prefer The Lancet more.
**As a clarification of terms, lung cancers are divided into two big groups: Small Cell Lung Carcinoma (SCLC) and Non-small cell lung carcinoma (NSCLC). You see where this is going. SCLC is capitalised throughout because it is highlighted as the deadliest subtype and there is no point in doing surgery even at the early phase since it's believed it's already spread across the body. Unlike other histological subtypes, it only has two grades: a. limited, and b. extensive. They are differentiated by whether the other hemithorax (other half of the chest cavity) is affected. For NSCLC, they are the most common and are less lethal than SCLC. Grading complies with the norm - TNM staging (standing for Tumour, Node [lymphatic], and Metastases [distant]).
***MPOWER refers to six components of the policy package: (1) monitoring tobacco use and prevention policies, (2) protection of people from tobacco smoke, (3) offer help to quit tobacco use, (4) warn about the dangers of tobacco use, (5) enforcement of bans on tobacco promotion and sponsorship, and (6) increased taxation on tobacco (thus increased retail prices).
References and Further Reading:
[1] Rafiemanesh H, Mehtarpour M, Khani F, et al. (2016). Epidemiology, incidence and mortality of lung cancer and their relationship with the development index in the world. Journal of thoracic disease, 8(6), 1094–1102. https://doi.org/10.21037/jtd.2016.03.91.
[2] Dela Cruz CS, Tanoue LT, Matthay RA. (2011). Lung cancer: epidemiology, etiology, and prevention. Clin Chest Med. 32(4):605-644. doi:10.1016/j.ccm.2011.09.001.
[3] Pesch B, Kendzia B, Gustavsson P, et al. (2012). Cigarette smoking and lung cancer--relative risk estimates for the major histological types from a pooled analysis of case-control studies. Int J Cancer. 131(5):1210-1219. doi:10.1002/ijc.27339.
[4] National Lung Screening Trial (NLST). National Cancer Institute. (2020). Retrieved 7 December 2020, from https://www.cancer.gov/types/lung/research/nlst.
[5] de Koning H, van der Aalst C, de Jong, et al. (2020). Reduced Lung-Cancer Mortality with Volume CT Screening in a Randomized Trial. New England Journal Of Medicine, 382(6), 503-513. https://doi.org/10.1056/nejmoa1911793.
[6] Gharraf H, Mehana S, ElNagar M. (2020). Role of CT in differentiation between subtypes of lung cancer; is it possible? The Egyptian Journal Of Bronchology, 14(1). https://doi.org/10.1186/s43168-020-00027-w.
[7] Garrido P, Viñolas N, Isla D, et al. (2019). Lung cancer in Spanish women: The WORLD07 project. European journal of cancer care, 28(1), e12941. https://doi.org/10.1111/ecc.12941.
[8] Kaur H, Sehgal IS, Bal A, et al. (2017). Evolving epidemiology of lung cancer in India: Reducing non-small cell lung cancer-not otherwise specified and quantifying tobacco smoke exposure are the key. Indian J Cancer. 54(1):285-290. doi:10.4103/ijc.IJC_597_16.
[9] Costa G, Thuler LC, Ferreira CG. (2016). Epidemiological changes in the histological subtypes of 35,018 non-small-cell lung cancer cases in Brazil. Lung Cancer. 97:66-72. doi:10.1016/j.lungcan.2016.04.019.
[10] Dubray J, Schwartz R, Chaiton M, et al. (2015). The effect of MPOWER on smoking prevalence. Tobacco Control. 24:540-542.
[11] Rodriguez-Lara V, Hernandez-Martinez JM, Arrieta O. (2018). Influence of estrogen in non-small cell lung cancer and its clinical implications. Journal of thoracic disease, 10(1), 482–497. https://doi.org/10.21037/jtd.2017.12.61.
[12] Chakraborty S, Ganti AK, Marr A, Batra SK. (2010). Lung cancer in women: role of estrogens. Expert review of respiratory medicine, 4(4), 509–518. https://doi.org/10.1586/ers.10.50.
[13] National Lung Screening Trial Research Team, Aberle DR, Adams AM, et al. (2011). Reduced lung-cancer mortality with low-dose computed tomographic screening. New England Journal of Medicine. 365(5):395-409. doi:10.1056/NEJMoa1102873.



Comments